For comprehensive support for your UK and Ireland clinical trials, turn to the Clinical suite from Pharma Intelligence. These powerful tools support your activities at every stage, from trial design through to trial disclosure. Individually, these tools provide superior data, critical to your decision making. Together, they form an end-to-end solution that gives you the competitive advantage throughout your clinical trial’s lifecycle.

Clinical suite flyer
Discover the comprehensive array of tools available to support your UK&I clinical trials. Move forward with confidence, from start to finish, with a full suite of solutions to support your Clinical trial planning, feasibility, awareness, recruitment, compliance, and disclosure needs.
Clinical solutions infographic
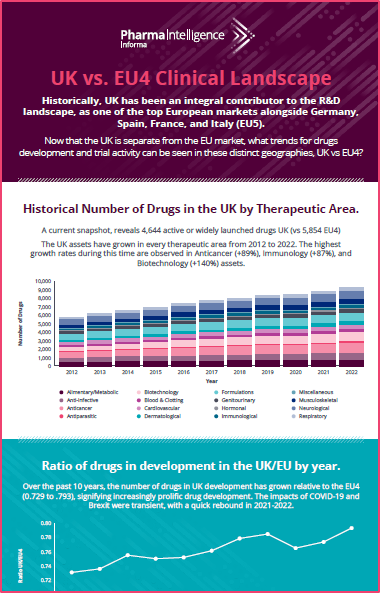
Gain a comprehensive view of the clinical trials landscape for UK and Ireland, with snapshots of all the current trials in progress by status, phase, disease, and therapeutic area, and compare these to the trials running in the top 5 European countries, using the industry’s most comprehensive, reliable and current global R&D intelligence suite of solutions.