Enhancements to
Citeline map visualizations
Several exciting enhancements were made to the Citeline map
visualizations to increase their overall value and usability. The
enhancements are summarized below:
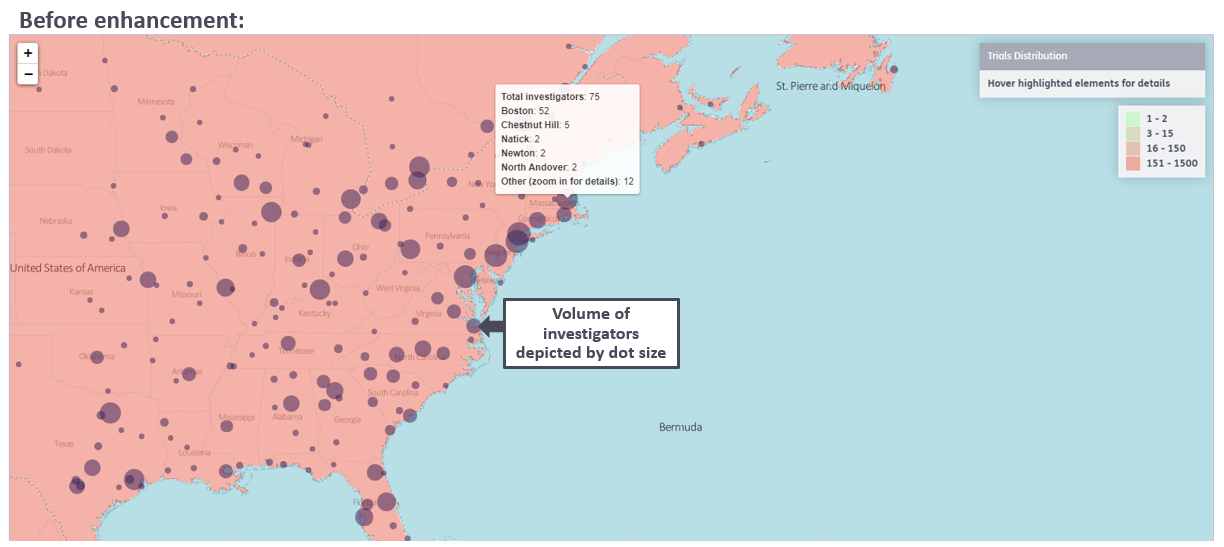
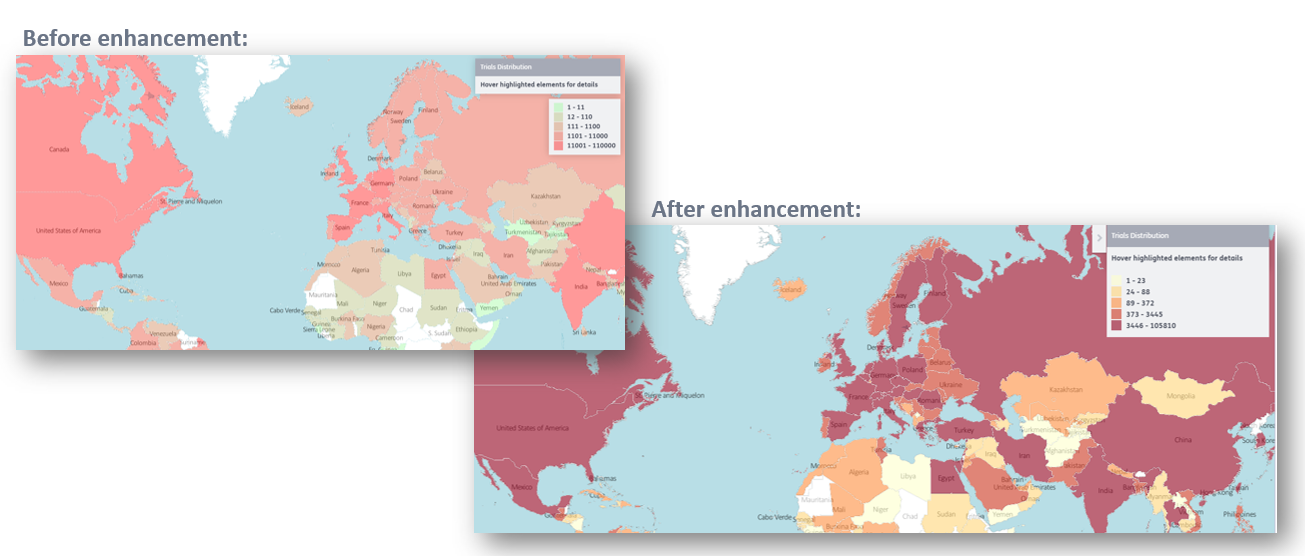
1. Distribution of heatmap data is clearer with more differentiated colors:
2. Ability to export the map visuals to JPEG or PNG for use in proposals, presentations, and reports to support decision making:
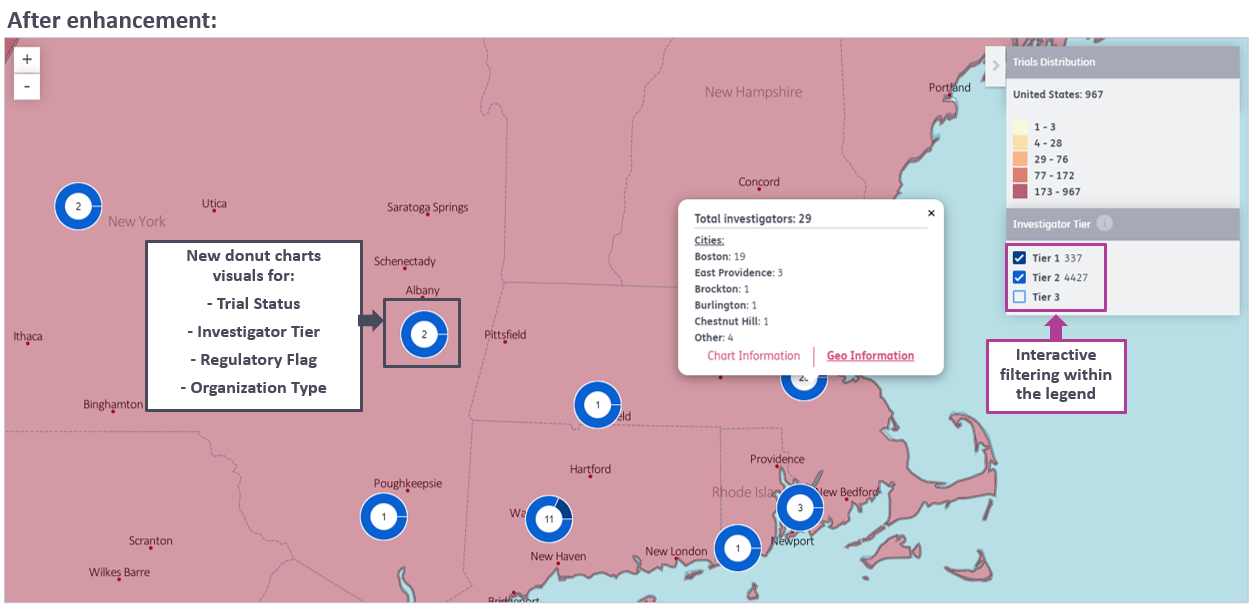
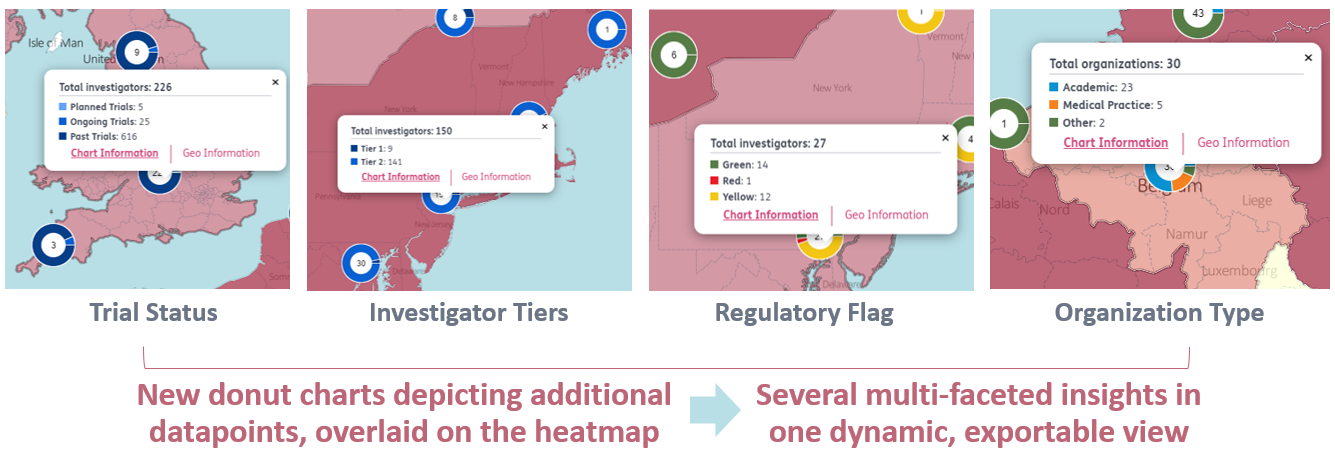
3. Addition of new datapoints and charts to the Sitetrove map to enable new popular use cases:
- Analyze multiple data points about investigators and organizations in one dynamic, interactive visual
- Evaluate locations and workload for a subset of investigators based on associated trial statuses – quickly
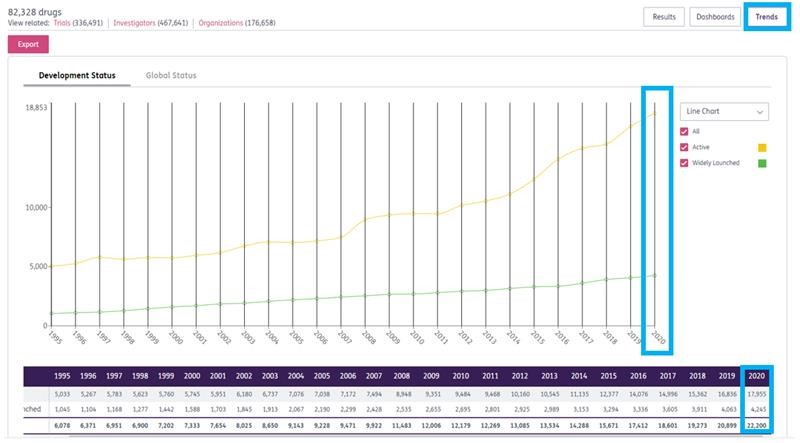
- Visualize the competitive landscape based on drug attributes, such as therapeutic class or mechanism of action